We will achieve the SDGs through the development of biopharmaceuticals.
Aiming for advanced analysis and high quality of biopharmaceuticals
Since the approval of recombinant human insulin in the United States in 1982, many biopharmaceuticals have been developed and used. Biopharmaceuticals generally have advantages such as fewer side effects, but they also have problems such as not being highly stable. We conduct advanced analysis of biopharmaceuticals and aim to improve their quality.

- What is biopharmaceuticals ?
-
Biopharmaceuticals are manufactured by applying biotechnologies such as genetic recombination and cell culture technologies, and use proteins and other substances as their active ingredients. Antibody drugs, which are representative biopharmaceuticals, are characterized by their high target specificity, resistance to adverse side reactions, and high efficacy.
 Prof.UCHIYAMA
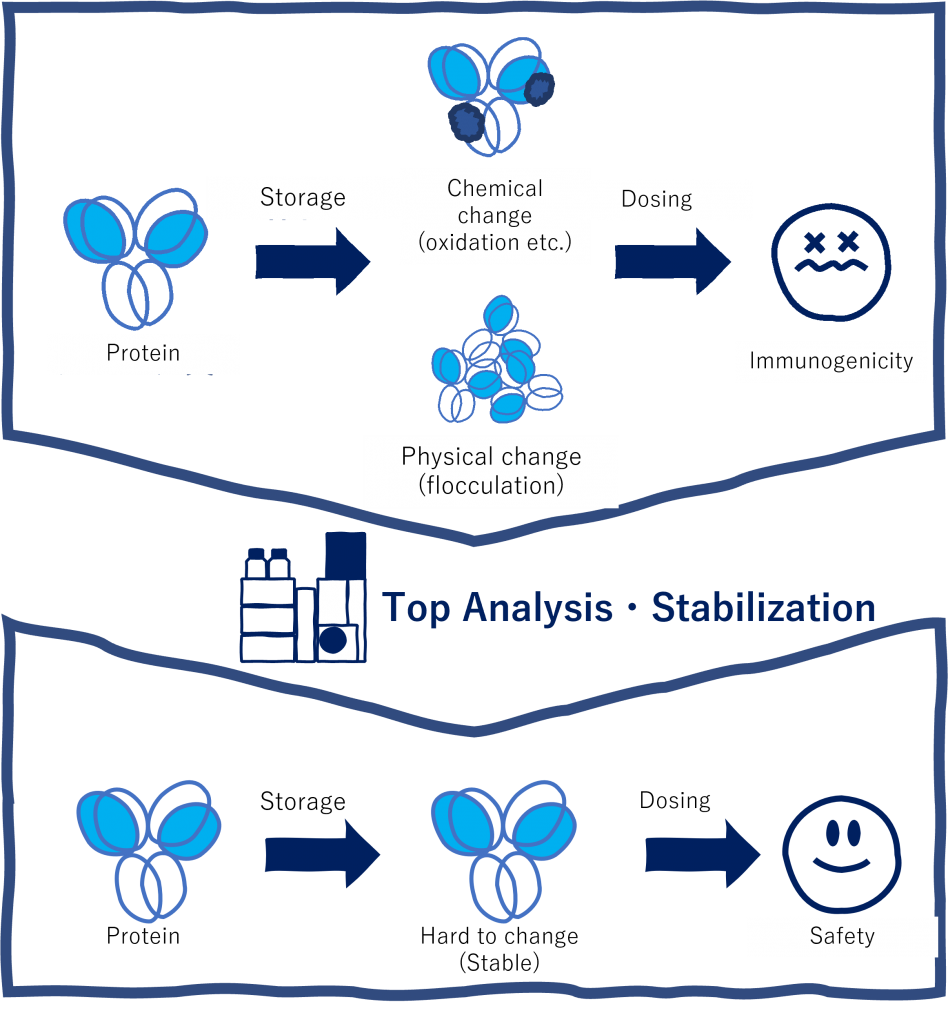
Prof.UCHIYAMAIt is not easy to maintain the properties of the active ingredient, protein, over a long period of time, and chemical and physical changes may occur during storage. During storage, chemical and physical changes may occur, and such changes may be immunogenic, reducing the efficacy of the administered biopharmaceutical or causing serious side effects. We have been conducting research on advanced analytical methods for biopharmaceuticals. In addition, we are developing methods to reduce the number of variants by using these advanced analytical methods.
- What is immunogenicity ?
-
A property that induces the production of antibodies and cellular immunity. If the biopharmaceutical variant is immunogenic, antibodies to the biopharmaceutical will be produced, leading to reduced drug efficacy and side effects.


Bringing innovation to drug safety through collaboration between industry, academia and government
Many joint research projects have been returned to society. We also participate in industry-academia-government joint research projects, and the results of our research are being used to create regulations for pharmaceuticals.



We are conducting joint industry-university research focusing not only on protein solutions but also on dosing devices. By improving the material of the dosing device, we hope to lead to the development of safer biopharmaceuticals.
- What is dosing devices ?
-
Biopharmaceuticals are often administered by injection or intravenous infusion, as they are degraded by enzymes in the digestive tract. Ensuring the stability of the protein in the administration device is also important for the development of high-quality biopharmaceuticals.
Working with cutting-edge analysis technology
In close cooperation with industry, we are engaged in research and development that utilizes reliable basic knowledge and the world’s most advanced measurement technology under a system that directly contributes to industry. An efficient and effective way of working is what our laboratory strives for.





By eliminating waste and creating a focused work environment, we aim to create a workplace that is not only rewarding, but also easy to work in.
← Mass Analysis for quadrupole


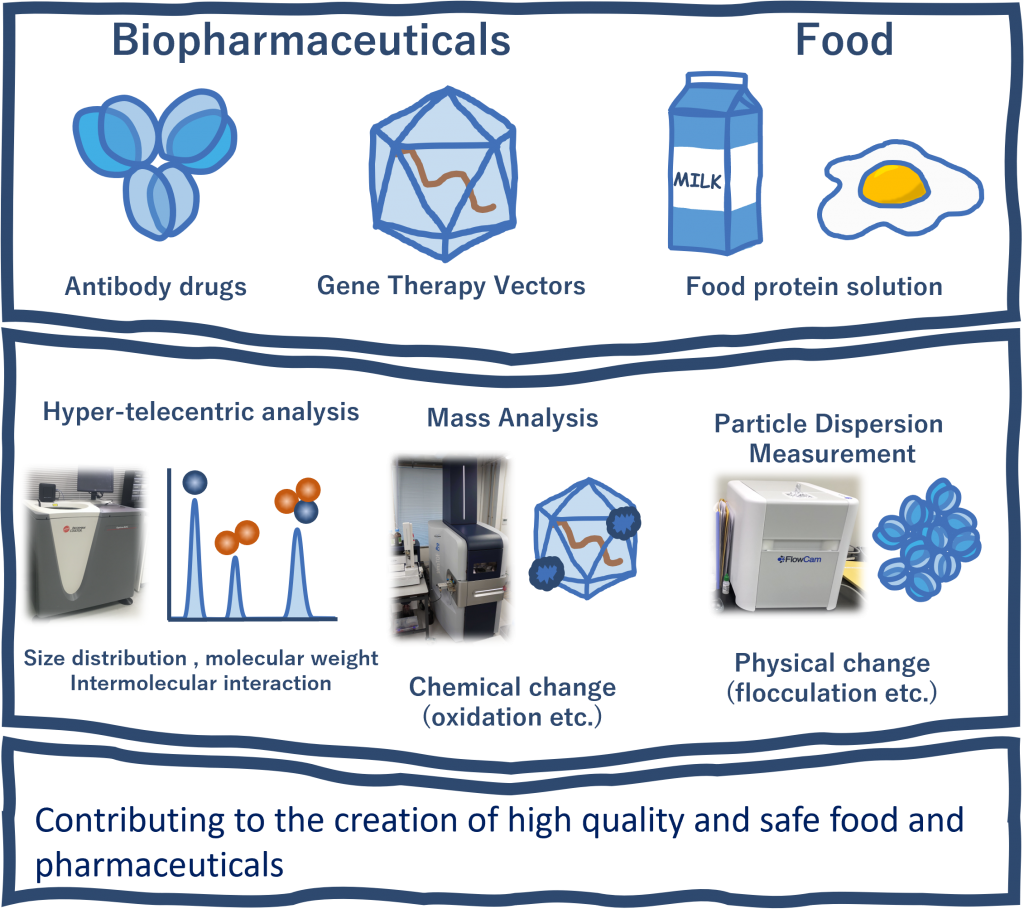
To supply safe and secure food
Our advanced analytical methods for antibody drugs, as well as our experience and measurement methods for improving quality, have been applied to food products, and we are also contributing to the field of food engineering.



Food is a complex system with many components. We are working to develop new research fields to understand and control complex systems using cutting-edge measurement technology.